Topic Objectives
By the end of this topic, the learner should be able to:
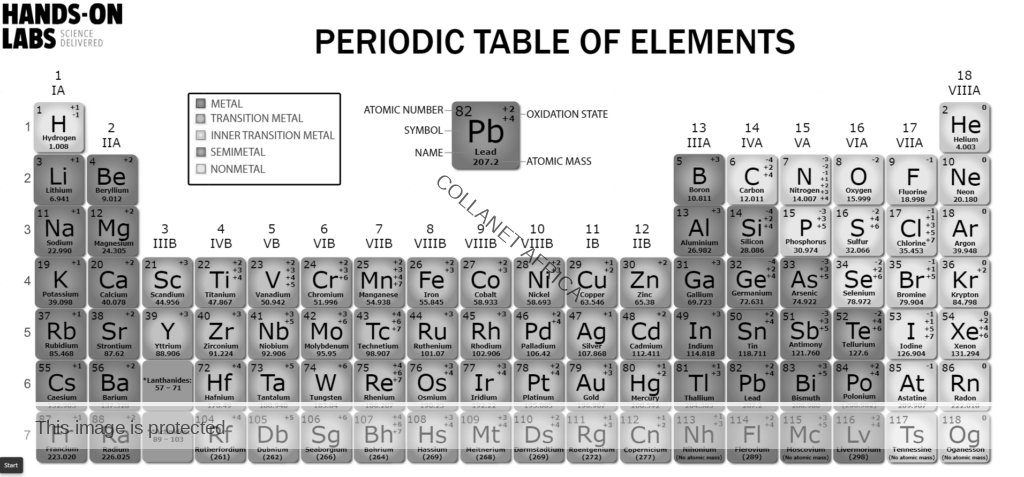
- Name and write the chemical symbols of the first twenty elements of the periodic table;
- Describe the structure of the atom and write the electron arrangement of the first twenty elements of the periodic table;
- Explain the electron arrangement of the atom in terms of energy levels;
- Define atomic number, mass number, isotopes and relative atomic mass;
- Calculate the relative atomic masses from isotopic composition;
- Explain the position of an element in the periodic table in terms of the electron arrangement;
- Define valency and oxidation number of an element;
- Predict the type of ion formed from a given electron arrangement of an atom;
- Predict the valencies and oxidation numbers from position of elements in the periodic table;
- Derive the formulae of some simple compounds from valencies of elements and radicals;
- Write simple balanced chemical equations.
Unit Content

Login
Accessing this unit requires a login. Please enter your credentials below!